Multiple Choice
Given the data in the table below,ΔH°rxn for the reaction PCl3 (g) + 3HCl (g) → 3Cl2 (g) + PH3 (g)
Is ________ kJ. 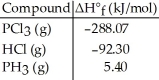
A) -570.37
B) -385.77
C) 570.37
D) 385.77
E) The ΔH°f of Cl2 (g) is needed for the calculation.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q111: For a given process at constant pressure,w
Q112: The specific heat capacity of liquid mercury
Q113: How much heat is released when 29.5
Q114: When 0.800 grams of NaOH is dissolved
Q115: The value of ΔH° for the reaction
Q117: Given the following reactions Fe<sub>2</sub>O<sub>3</sub> (s)+ 3CO
Q118: Which one of the following is an
Q119: ΔH for an endothermic process is _
Q120: The value of ΔH° for the reaction
Q121: Which of the following is a statement