Multiple Choice
The value of ΔE for a system that performs 139 kJ of work on its surroundings and gains 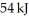 of heat is
of heat is 
A) -85
B) 193
C) 7506
D) 85
E) -193
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q98: The kinetic energy of a 10.3 g
Q99: At what velocity (m/s)must a <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg"
Q100: A 50.0-g sample of liquid water at
Q101: The term standard conditions with respect to
Q102: Which one of the following statements is
Q104: A 23.2 g piece of space debris
Q105: Given the following reactions N<sub>2</sub> (g)+ O<sub>2</sub>
Q106: A chemical reaction that absorbs heat from
Q107: The energy released by combustion of _
Q108: Calculate the kinetic energy in joules of