Multiple Choice
Calculate the work (kJ) done during a reaction in which the internal volume expands from 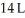 to
to 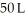 against a vacuum (an outside pressure of
against a vacuum (an outside pressure of 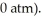
A) 0; kJ No work is done.
B) 3.6 kJ
C) -3.6 kJ
D) 6.5 kJ
E) -6.5 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q59: Of the following,ΔH°<sub>f</sub> is not zero for
Q60: The temperature of a 24.3 g sample
Q61: A 22.9 g sample of iron absorbs
Q62: A 5.00-g sample of copper metal at
Q63: What is the kinetic energy of a
Q65: Given the data in the table below,ΔH°<sub>rxn</sub>
Q66: Objects can possess energy as _. (a)endothermic
Q67: For the following reactions,the ΔH°<sub>rxn</sub> is NOT
Q68: For the following reactions,the ΔH°<sub>rxn</sub> is NOT
Q69: Which one of the following conditions would