Multiple Choice
Calculate the work (kJ) done during a reaction in which the internal volume contracts from 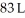 to
to 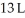 against an outside pressure of
against an outside pressure of 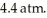
A) 31 kJ
B) 43 kJ
C) -31 kJ
D) -43 kJ
E) 0 kJ; No work is done.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q138: The units of specific heat are _.<br>A)K/J
Q139: The value of ΔE for a system
Q140: The internal energy can be increased by
Q141: When _ is constant,the enthalpy change of
Q142: One joule equals 1 kg-m<sup>2</sup>/s<sup>2</sup>.
Q144: A _ ΔH corresponds to an _
Q145: Of the following,which one is a state
Q146: CH<sub>3</sub>OH (l)decomposes into carbon monoxide and hydrogen
Q147: Calculate the kinetic energy in J of
Q148: Given the data in the table below,ΔH°