Multiple Choice
The combustion of titanium with oxygen produces titanium dioxide: Ti (s) + O2(g) → TiO2 (s)
When 0.610 g of titanium is combusted in a bomb calorimeter,the temperature of the calorimeter increases from 25.00 °C to 50.50 °C.In a separate experiment,the heat capacity of the calorimeter is measured to be 9.84 kJ/K.The heat of reaction for the combustion of a mole of Ti in this calorimeter is ________ kJ/mol.
A) 2.09
B) 4.14
C) -311
D) -0.154
E) -1.98 × 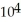
Correct Answer:

Verified
Correct Answer:
Verified
Q26: The kinetic energy of a 7.3 kg
Q27: The value of ΔH° for the reaction
Q28: The change in the internal energy of
Q29: The ΔH<sub>vap </sub>of water is 40.7 kJ
Q30: Given the following reactions H<sub>2</sub>O (l)→ H<sub>2</sub>O
Q32: The value of ΔH° for the reaction
Q33: The _ of a reaction is the
Q34: What is the molar heat capacity (in
Q35: The ΔH<sub>vap </sub>of water is 40.7 kJ
Q36: The value of ΔH° for the reaction