Multiple Choice
The specific heat capacity of liquid water is 4.18 J/g-K.How many joules of heat are needed to raise the temperature of 7.25 g of water from 20.0 °C to 44.1 °C?
A) 41.8 J
B) 730 J
C) 1.94 × 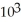 J
J
D) 2.39 × 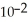 J
J
E) 66.8 J
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q126: Hydrogen peroxide decomposes to water and oxygen
Q127: ΔH for the reaction IF<sub>5</sub> (g)→ IF<sub>3
Q128: Coal contains hydrocarbons of high molecular weight
Q129: An 8 oz.bottle of energy drink contains
Q130: The ΔH<sub>rxn </sub>for the combustion of methane
Q132: The specific heat capacity of lead is
Q133: The most abundant fossil fuel is _.<br>A)natural
Q134: The value of ΔH° for the reaction
Q135: Given the following reactions N<sub>2</sub> (g)+ O<sub>2</sub>
Q136: Carbon monoxide and oxygen gas react to