Multiple Choice
A 4.50-g sample of liquid water at 25.0 °C is heated by the addition of 133 J of energy.The final temperature of the water is ________ °C.The specific heat capacity of liquid water is 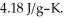
A) 149
B) 25.1
C) -17.9
D) 32.1
E) 7.07
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q66: Objects can possess energy as _. (a)endothermic
Q67: For the following reactions,the ΔH°<sub>rxn</sub> is NOT
Q68: For the following reactions,the ΔH°<sub>rxn</sub> is NOT
Q69: Which one of the following conditions would
Q70: A slice of cake contains 29.0 grams
Q72: The kinetic energy of a 23.2-g object
Q73: A 10.1 g sample of NaOH is
Q74: Given the following reactions CaCO<sub>3</sub> (s)→ CaO
Q75: Fuel values of hydrocarbons increase as the
Q76: What is the enthalpy change (in kJ)of