Multiple Choice
Which one of the following is the correct electron configuration for a ground-state nitrogen atom?
A) 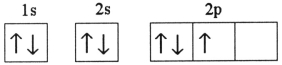
B) 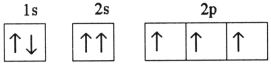
C) 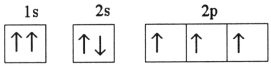
D) 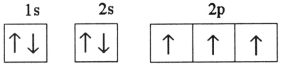
E) None of the above is correct.
Correct Answer:

Verified
Correct Answer:
Verified
Q138: The frequency of electromagnetic radiation required to
Q139: What is the energy of a photon
Q140: The maximum angular momentum quantum number in
Q141: Which electron orbital diagram is written correctly
Q142: The n = 1 shell contains _
Q144: What is the frequency (s<sup>-1</sup>)of electromagnetic radiation
Q145: When the electron in a hydrogen atom
Q146: The ground-state configuration of fluorine is _.<br>A)[He]2s<sup>2</sup>2p<sup>2</sup><br>B)[He]2s<sup>2</sup>2p<sup>3</sup><br>C)[He]2s<sup>2</sup>2p<sup>4</sup><br>D)[He]2s<sup>2</sup>2p<sup>5</sup><br>E)[He]2s<sup>2</sup>2p<sup>6</sup>
Q147: The energy of a photon that has
Q148: The ground-state configuration of tungsten is _.<br>A)[Ar]4s<sup>2</sup>3d<sup>3</sup><br>B)[Xe]6s<sup>2</sup>4f<sup>14</sup>5d<sup>4</sup><br>C)[Ne]3s<sup>1</sup><br>D)[Xe]6s<sup>2</sup>4f<sup>7</sup><br>E)[Kr]5s<sup>2</sup>4d<sup>10</sup>5p<sup>5</sup>