Multiple Choice
Which electron configuration denotes an atom in its ground state?
A) 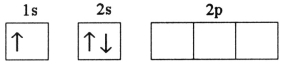
B) 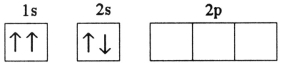
C) 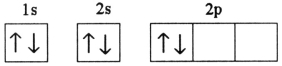
D) 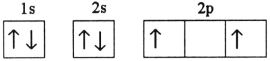
E) 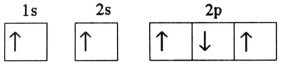
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q149: The energy of a photon that has
Q150: The electron density of the 5s orbital
Q151: A 34.0 mg object with a de
Q152: What is the correct ground state electron
Q153: A line spectrum contains radiation of _
Q155: An electron in a Bohr hydrogen atom
Q156: The ground state electron configuration of copper
Q157: Which one of the following orbitals can
Q158: If a hydrogen atom electron jumps from
Q159: The angular momentum quantum number is 3