Multiple Choice
Using Bohr's equation for the energy levels of the electron in the hydrogen atom,determine the 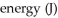 of an electron in the n = 4 level.
of an electron in the n = 4 level.
A) -1.36 × 10-19
B) -5.45 × 10-19
C) -7.34 × 1018
D) -1.84 × 10-29
E) +1.84 × 10-29
Correct Answer:

Verified
Correct Answer:
Verified
Q114: At maximum,an d-subshell can hold _ electrons.<br>A)10<br>B)6<br>C)2<br>D)8<br>E)14
Q115: Which orbital is degenerate with a 3d<sub>z</sub><sup>2</sup>
Q116: The element that corresponds to the electron
Q117: The element that has a valence configuration
Q118: How many quantum numbers are necessary to
Q120: What is the wavelength of light (nm)that
Q121: The energy of a photon of light
Q122: The valence shell of the element X
Q123: The element that has a valence configuration
Q124: At what speed (m/s)must a 10.0-mg object