Multiple Choice
Electromagnetic radiation with a wavelength of 641 nm appears as orange light to the human eye.The energy of one photon of this light is 3.10 × 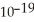 J.Thus,a laser that emits
J.Thus,a laser that emits 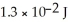 of energy in a pulse of light at this wavelength produces ________ photons in each pulse.
of energy in a pulse of light at this wavelength produces ________ photons in each pulse.
A) 2.4 × 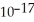
B) 6.3 × 10-24
C) 2.7 × 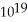
D) 4.2 × 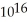
E) 6.5 × 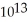
Correct Answer:

Verified
Correct Answer:
Verified
Q15: An electron cannot have the quantum numbers
Q16: An NMR spectrum results from photon irradiation
Q17: Which one of the following is considered
Q18: The ground-state electron configuration of the element
Q19: Electromagnetic radiation travels through vacuum at a
Q21: The wavelength of an electron whose velocity
Q22: The angular momentum quantum number (l)value of
Q23: What is the frequency of light (s<sup>-1</sup>)that
Q24: The wavelength of a photon that has
Q25: The condensed electron configuration of argon,element 18,is