Multiple Choice
The complete electron configuration of sulfur,element 16,is ________.
A) 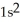
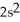
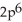 3s23p4
3s23p4
B) 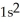
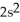 2p103s2
2p103s2
C) 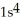
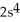 2p63s2
2p63s2
D) 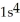 2s42p8
2s42p8
E) 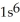 2s62p23s2
2s62p23s2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q123: The element that has a valence configuration
Q124: At what speed (m/s)must a 10.0-mg object
Q125: [Ne]3s<sup>2</sup>3p<sup>3</sup> is the electron configuration of a(n)_
Q126: The wavelength of light emitted from a
Q127: How many p-orbitals are occupied in a
Q129: A mole of red photons of wavelength
Q130: The first shell in the ground state
Q131: What is the principal quantum number for
Q132: Which of the subshells below do not
Q133: [Ar]4s<sup>2</sup>3d<sup>10</sup>4p<sup>3</sup> is the electron configuration of a(n)_