Multiple Choice
The complete electron configuration of vanadium,element 23,is ________.
A) 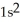
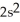
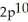
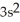 3p7
3p7
B) 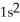
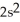
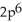
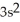
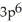 3d34s2
3d34s2
C) 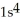
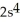
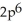
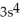 3p5
3p5
D) 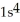
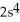
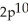 3s43p1
3s43p1
E) 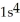
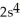
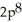
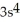 3p3
3p3
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q35: Which of the following is a valid
Q36: Calculate the longest wavelength of light (nm)that
Q37: How many different principal quantum numbers can
Q38: The energy of a photon that has
Q39: The energy of a photon that has
Q41: A transition in the Bohr hydrogen atom
Q42: When the value of n is greater
Q43: A spectrum containing only _ wavelengths is
Q44: The 4d subshell in the ground state
Q45: An electron cannot have the quantum numbers