Multiple Choice
Which isoelectronic series is correctly arranged in order of increasing radius?
A) K+ < Ca2+ < Ar < Cl-
B) 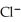 < Ar < K+ < Ca2+
< Ar < K+ < Ca2+
C) Ca2+ < Ar < K+ < Cl-
D) Ca2+ < K+ < Ar < Cl-
E) Ca2+ < K+ < Cl- < Ar
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q9: Cl<sub>2</sub> (g)+ H<sub>2</sub>O (l)→ _<br>A)HCl (aq)+ HOCl
Q10: Of the following elements,_ has the most
Q11: What is the coefficient of Na when
Q12: The difference in metallic character is the
Q13: Which of the following metals differ in
Q15: Of the following,which gives the correct order
Q16: Element M reacts with oxygen to form
Q17: A group of ions all containing the
Q18: Of the following,which gives the correct order
Q19: Which one of the following has the