Multiple Choice
The formal charge on carbon in the molecule below is ________. 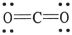
A) 0
B) +1
C) +2
D) +3
E) -1
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q37: What is the electron configuration for the
Q38: The strength of a _ bond is
Q39: Which of the following noble gas electron
Q40: A positive change in bond enthalpy is
Q41: As the number of covalent bonds between
Q43: The formula of palladium (IV)sulfide is _.<br>A)Pd<sub>2</sub>S<sub>4</sub><br>B)PdS<sub>4</sub><br>C)Pd<sub>4</sub>S<br>D)PdS<sub>2</sub><br>E)Pd<sub>2</sub>S<sub>2</sub>
Q44: In the molecule below,which atom has the
Q45: A triple bond consists of _ pairs
Q46: A double bond consists of _ pairs
Q47: The most electronegative atom of the ones