Multiple Choice
The formal charge on sulfur in SO42- is ________,where the Lewis structure of the ion is: 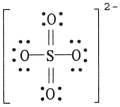
A) -2
B) 0
C) +2
D) +4
E) -4
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q6: The _ ion has eight valence electrons.<br>A)Sc<sup>3+</sup><br>B)Ti<sup>3+</sup><br>C)V<sup>3+</sup><br>D)Cr<sup>3+</sup><br>E)Mn<sup>3+</sup>
Q7: In which of the molecules below is
Q8: Given the electronegativities below,which covalent single bond
Q9: Which of the following would have to
Q10: In the Lewis structure of HCO<sub>3</sub><sup>-</sup>,the formal
Q12: Ni<sup>2+</sup> ions are represented by the electron
Q13: Of the bonds below,_ is the least
Q14: Which of the following Lewis structures would
Q15: Which of the following would have to
Q16: Determining lattice energy from Born-Haber cycle data