Multiple Choice
Using the table of bond dissociation energies,the ΔH for the following reaction is ________ kJ. 2HCl (g) + F2 (g) → 2HF (g) + Cl2 (g) 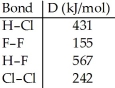
A) -359
B) -223
C) 359
D) 223
E) 208
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q78: For _ forms of a molecule or
Q79: Using the table of average bond energies
Q80: Give the electron configuration of Zn<sup>2+</sup>.
Q81: What is the maximum number of double
Q82: Draw the Lewis structure of ICl<sub>2</sub><sup>+</sup>.
Q84: Atoms surrounded by eight valence electrons tend
Q85: Bond enthalpy is _.<br>A)always positive<br>B)always negative<br>C)sometimes positive,
Q86: Based on the octet rule,boron will most
Q87: As electronegativity difference increases,bond length will decrease.
Q88: How many equivalent resonance structures can be