Multiple Choice
Of the following molecules,only ________ is polar.
A) BeCl2
B) BF3
C) C 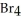
D) SiH2Cl2
E) Cl2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q157: Using the VSEPR model,the molecular geometry of
Q158: The N-H bond in ammonia consists of
Q159: Three monosulfur fluorides are observed: SF<sub>2</sub>,SF<sub>4</sub>,and SF<sub>6</sub>.Of
Q160: What are the three bond angles in
Q161: _ hybrid orbitals are used for bonding
Q163: The electron-domain geometry of the AsF<sub>6</sub><sup>-</sup> ion
Q164: In molecular orbital theory,the bond order of
Q165: The molecular geometry of the H<sub>3</sub>O<sup>+</sup> ion
Q166: The Cl-Si-Cl bond angle in the SiCl<sub>2</sub>F<sub>2</sub>
Q167: There are _ σ and _ π