Multiple Choice
What is the hybridization of the carbon atom attached to the two oxygen atoms in the structure below? 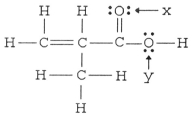
A) sp3
B) sp
C) sp3d2
D) sp2
E) sp3d
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q22: For a molecule with the formula AB<sub>3</sub>,the
Q23: Valence bond theory addresses all of the
Q24: When four atomic orbitals are mixed to
Q25: Of the following species,_ will have bond
Q26: The hybridization of orbitals on the central
Q28: Of the following,the central atom is sp<sup>3</sup>d<sup>2</sup>
Q29: The highest energy occupied molecular orbital in
Q30: In comparing the same two atoms bonded
Q31: In a SO<sub>4</sub><sup>2-</sup> ion,"localized" bonding electrons are
Q32: The combination of two atomic orbitals results