Multiple Choice
The hybridization of the oxygen atom labeled x in the structure below is ________. 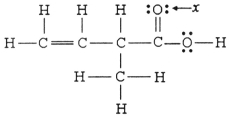
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
Correct Answer:

Verified
Correct Answer:
Verified
Q170: In order to produce sp<sup>3 </sup>hybrid orbitals,_
Q171: The molecular geometry of the CHCl<sub>3</sub> molecule
Q172: Which of the molecules has a square
Q173: The hybridization of nitrogen in the H-C
Q174: The π bond in ethylene,H<sub>2</sub>C <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg"
Q176: The electron-domain geometry of _ is tetrahedral.<br>A)CBr<sub>4</sub><br>B)PH<sub>3</sub><br>C)CCl<sub>2</sub>Br<sub>2</sub><br>D)XeF<sub>4</sub><br>E)all
Q177: Construct a molecular orbital diagram for a
Q178: There is/are _ σ bond(s)in the molecule
Q179: Of the following molecules,only _ is polar.<br>A)CCl<sub>4</sub><br>B)BCl<sub>3</sub><br>C)NCl<sub>3</sub><br>D)BeCl<sub>2</sub><br>E)Cl<sub>2</sub>
Q180: Mixing one s atomic orbital and one