Multiple Choice
There is/are ________ π bond(s) in the molecule below. 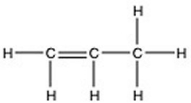
A) 7
B) 6
C) 2
D) 1
E) 0
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q56: A typical triple bond consists of _
Q57: The hybridizations of nitrogen in NF<sub>3</sub> and
Q58: The carbon-hydrogen σ bond in ethylene,H<sub>2</sub>C <img
Q59: In which of the molecules is the
Q60: A _ compound would display unpaired electrons
Q62: How many unhybridized p atomic orbital(s)are found
Q63: Structural changes around a _ bond in
Q64: Which of the following molecules or ions
Q65: There are _ σ bonds and _
Q66: The bond order of a homonuclear diatomic