Multiple Choice
In ideal gas equation calculations,expressing pressure in Pascals (Pa) ,necessitates the use of the gas constant,R,equal to ________.
A) 0.08206 atm L mol-1K-1
B) 8.314 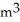 -Pa/mol-K
-Pa/mol-K
C) 62.36 L torr mol-1K-1
D) 1.987 cal mol-1K-1
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q64: What volume (mL)of fluorine gas is required
Q65: A sample of gas (1.9 mol)is in
Q66: A sample of a gas originally at
Q67: What is the density of chlorine gas
Q68: How many liters of CO<sub>2</sub> are formed
Q70: The kinetic-molecular theory predicts that pressure rises
Q71: Real gases do not behave ideally at
Q72: A tank containing both HF and HBr
Q73: According to the kinetic-molecular theory,molecules of different
Q74: The van der Waals equation for real