Multiple Choice
Using the van der Waals equation,the pressure in a 22.4 L vessel containing 1.00 mol of neon gas at 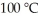 is ________ atm. (a = 0.211 L2-atm/mol2,b = 0.0171 L/mol)
is ________ atm. (a = 0.211 L2-atm/mol2,b = 0.0171 L/mol)
A) 0.730
B) 1.00
C) 1.21
D) 1.37
E) 0.367
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q10: Automobile air bags use the decomposition of
Q11: The pressure exerted by 1.0 mol of
Q12: A 0.133 mol sample of gas in
Q13: A helium balloon is filled to a
Q14: CO (5.00 g)and CO<sub>2</sub> (5.00 g)were placed
Q16: CO (5.00 g)and CO<sub>2</sub> (5.00 g)were placed
Q17: A sample of gas (24.2 g)initially at
Q18: One significant difference between gases and liquids
Q19: What is the volume (in m<sup>3</sup>)of a
Q20: Arrange the following gases in order of