Multiple Choice
A fixed amount of gas at 25.0 °C occupies a volume of 8.66 L when the pressure is 629 torr.Use Charles's law to calculate the volume (L) the gas will occupy when the temperature is increased to 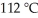 while maintaining the pressure at 629 torr.
while maintaining the pressure at 629 torr.
A) 9.26
B) 11.2
C) 1.93
D) 6.70
E) 38.8
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q50: 5.25 g of zinc metal reacts with
Q51: The density of HCN is _ g/L
Q52: A 5.50 L vessel contains 0.348 mol
Q53: What is the density of (g/L)CO<sub>2</sub> at
Q54: A pressure vessel contains CO<sub>2</sub> (P<sub>CO2</sub> =
Q56: A 255 mL round-bottom flask is weighed
Q57: A sample of Ne gas (2.5 L)at
Q58: A pressure of 0.500 atm is the
Q59: A 0.325 L flask filled with gas
Q60: What is the temperature at STP?<br>A)0 °C<br>B)25