Multiple Choice
Calcium hydride ( 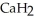 ) reacts with water to form hydrogen gas:
) reacts with water to form hydrogen gas: 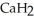 (s) +
(s) + 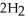 O (l) →
O (l) → 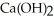 (aq) +
(aq) + 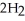 (g) How many grams of
(g) How many grams of 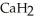 are needed to generate 48.0 L of
are needed to generate 48.0 L of 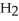 gas at a pressure of 0.995 atm and a temperature of 32 °C?
gas at a pressure of 0.995 atm and a temperature of 32 °C?
A) 56.8
B) 0.954
C) 161
D) 40.1
E) 80.3
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q147: Which noble gas is expected to show
Q148: The volume of 0.15 mol of an
Q149: Standard temperature and pressure (STP),in the context
Q150: What volume (L)of NH<sub>3</sub> gas at STP
Q151: How much CO<sub>2</sub> (L)is produced when 2.10
Q153: A real gas will behave most like
Q154: A gas in a 57.1 L pressure
Q155: Which one of the following gases would
Q156: Which of the following is not possible
Q157: What is the rms speed (m/s)of NO<sub>2</sub>