Multiple Choice
The enthalpy change for converting 1.00 mol of ice at -25.0 °C to water at 50.0 °C is  The specific heats of ice,water,and steam are
The specific heats of ice,water,and steam are 
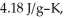 and
and 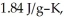 respectively.For
respectively.For 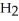 O,Δ
O,Δ 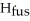 = 6.01 kJ/mol,and
= 6.01 kJ/mol,and 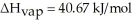 .
.
A) 12.28
B) 6.27
C) 10.71
D) 4709
E) 8.83
Correct Answer:

Verified
Correct Answer:
Verified
Q105: The property responsible for the "beading up"
Q106: Which of the following has London dispersion
Q107: On a phase diagram,the critical pressure is
Q108: Which molecule is the least volatile?<br>A)CH<sub>3</sub>Cl<br>B)CH<sub>3</sub>I<br>C)CH<sub>3</sub>F<br>D)CH<sub>4</sub><br>E)CH<sub>3</sub>Br
Q109: - <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="- is
Q111: At 1 atm,an unknown sample melts at
Q112: Which one of the following derivatives of
Q113: Hydrogen bonding is a special case of
Q114: In the _ liquid crystalline phase,the component
Q115: On the phase diagram shown above,segment _