Multiple Choice
The enthalpy change for converting 10.0 g of ice at -50.0 °C to water at 50.0 °C is ________ kJ.The specific heats of ice,water,and steam are 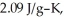
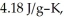 and
and 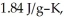 respectively.For
respectively.For 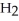 O,Δ
O,Δ 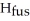 = 6.01 kJ/mol,and
= 6.01 kJ/mol,and 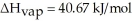 .
.
A) 12.28
B) 4.38
C) 3138
D) 6.47
E) 9.15
Correct Answer:

Verified
Correct Answer:
Verified
Q11: Which compound has the strongest intermolecular forces?<br>A)CCl<sub>4</sub><br>B)CI<sub>4</sub><br>C)CH<sub>4</sub><br>D)H<sub>2</sub><br>E)O<sub>2</sub>
Q12: _ liquid crystals are colored and change
Q13: Which of the following molecules has London
Q14: On the phase diagram shown above,the coordinates
Q15: What is the predominant intermolecular force in
Q17: Based on the following information,which compound has
Q18: The principal source of the difference in
Q19: _ is the energy required to expand
Q20: With what compound will NH<sub>3</sub> experience only
Q21: Under ordinary conditions,a substance will sublime rather