Multiple Choice
The heat of fusion of water is 6.01 kJ/mol.The heat capacity of liquid water is 75.3 J/mol ∙ K.The conversion of 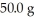 of ice at 0.00 °C to liquid water at 0.00°C requires ________ kJ of heat.
of ice at 0.00 °C to liquid water at 0.00°C requires ________ kJ of heat.
A) 6.01
B) 16.7
C) 75.3
D) 17.2
E) Insufficient data are given.
Correct Answer:

Verified
Correct Answer:
Verified
Q58: The heating curve shown was generated by
Q59: Crystalline solids _.<br>A)have their particles arranged randomly<br>B)have
Q60: When KBr dissolves in water,aqueous K<sup>+</sup> and
Q61: The strongest interparticle attractions exist between particles
Q62: The heating curve shown was generated by
Q64: London Dispersion Forces tend to _ in
Q65: A _ liquid crystal has the least
Q66: The initial discovery of a liquid crystal
Q67: The substance with the largest heat of
Q68: Of the following,_ should have the highest