Multiple Choice
Calculate the enthalpy change (in kJ) associated with the conversion of 25.0 grams of ice at -4.00 °C to water vapor at 109.0 °C.The specific heats of ice,water,and steam are 2.09 J/g-K,4.18 J/g-K,and 1.84 J/g-K,respectively.For H2O,Δ 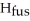 = 6.01 kJ/mol and Δ
= 6.01 kJ/mol and Δ 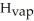 = 40.67 kJ/mol.
= 40.67 kJ/mol.
A) 64.8
B) 75.9
C) 11100
D) 12000
E) 112
Correct Answer:

Verified
Correct Answer:
Verified
Q114: In the _ liquid crystalline phase,the component
Q115: On the phase diagram shown above,segment _
Q116: The phase diagram of a substance is
Q117: Which molecule has hydrogen bonding as the
Q118: How high a liquid will rise up
Q119: The phase diagram of a substance is
Q121: Which one of the following substances will
Q122: The boiling points of normal hydrocarbons are
Q123: _ are particularly polarizable.<br>A)Small nonpolar molecules<br>B)Small polar
Q124: Heat of sublimation can be approximated by