Multiple Choice
Ethanol ( 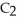
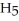 OH) melts at -114 °C.The enthalpy of fusion is 5.02 kJ/mol.The specific heats of solid and liquid ethanol are 0.97 J/g-K and 2.3 J/g-K,respectively.How much heat (kJ) is needed to convert 25.0 g of solid ethanol at -135 °C to liquid ethanol at -60 °C?
OH) melts at -114 °C.The enthalpy of fusion is 5.02 kJ/mol.The specific heats of solid and liquid ethanol are 0.97 J/g-K and 2.3 J/g-K,respectively.How much heat (kJ) is needed to convert 25.0 g of solid ethanol at -135 °C to liquid ethanol at -60 °C?
A) 207.3
B) -13.3
C) 6.34
D) 3617
E) 8.63
Correct Answer:

Verified
Correct Answer:
Verified
Q29: Which species has London dispersion forces as
Q30: Ethanol melts at -114 °C and boils
Q31: On the phase diagram shown above,the coordinates
Q32: The vapor pressure of a liquid _.<br>A)increases
Q33: Which one of the following substances will
Q35: Which of the following statements is false?<br>A)The
Q36: As a gaseous element condenses,the atoms become
Q37: Heats of vaporization are greater than heats
Q38: The predominant intramolecular force in CaBr<sub>2</sub> is
Q39: Based on molecular mass and dipole moment