Multiple Choice
A solution contains 15 ppm of benzene.The density of the solution is 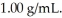 This means that ________.
This means that ________.
A) there are 15 mg of benzene in 1.0 g of this solution
B) 100 g of the solution contains 15 g of benzene
C) 1.0 g of the solution contains 15 × 10-6 g of benzene
D) 1.0 L of the solution contains 15 g of benzene
E) the solution is 15% by mass of benzene
Correct Answer:

Verified
Correct Answer:
Verified
Q97: Of the concentration units below,only _ uses
Q98: What is the molal concentration of a
Q99: What is the mole fraction of LiCl
Q100: A 2.05 m aqueous solution of some
Q101: The magnitudes of K<sub>f</sub> and of K<sub>b</sub>
Q103: On a clear day at sea level,with
Q104: Which of the following substances is least
Q105: An unsaturated solution is one that _.<br>A)has
Q106: An acetic acid aqueous solution contains 22%
Q107: A solution is prepared by dissolving 27.7