Multiple Choice
What is the concentration (M) of HCl in a solution that is prepared by dissolving 25.5 g of HCl in 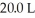 of water? (Assume volume does not change after adding HCl.)
of water? (Assume volume does not change after adding HCl.)
A) 1.28
B) 0.0350
C) 35.0
D) 3.50 × 10-5
E) 14.0
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q36: What is the molality of LiCl in
Q37: What is the molarity of phosphoric acid
Q38: Which of the following liquids will have
Q39: The value of the boiling-point-elevation constant (K<sub>b</sub>)depends
Q40: The process of a substance sticking to
Q42: What is the mass % of ammonium
Q43: Adding a nonvolatile solute to a solution
Q44: A solution is prepared by adding 40.00
Q45: Which one of the following substances is
Q46: What is the mole fraction of hydrochloric