Multiple Choice
Which one of the following substances would be the most soluble in C 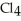 ?
?
A) C4H10
B) Li2O
C) NH3
D) HCl
E) CH3CH2CH2OH
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q89: Molality is defined as the _.<br>A)moles solute/moles
Q90: Of the following,a 0.1 M aqueous solution
Q91: A solution with a concentration higher than
Q92: An aqueous solution with a concentration of
Q93: Which of the following liquids will have
Q95: A solution containing 10.0 g of an
Q96: The mole fraction of urea (MW =
Q97: Of the concentration units below,only _ uses
Q98: What is the molal concentration of a
Q99: What is the mole fraction of LiCl