Multiple Choice
The solubility of oxygen gas in water at 25 °C and 1.0 atm pressure of oxygen is 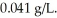 The solubility of oxygen in water at 4.0 atm and 25 °C is ________ g/L.
The solubility of oxygen in water at 4.0 atm and 25 °C is ________ g/L.
A) 0.041
B) 0.014
C) 0.31
D) 0.16
E) 4.0
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q150: The most likely van't Hoff factor for
Q151: The concentration of urea (MW = 60.0
Q152: Hydrophobic colloids _.<br>A)are those that contain water<br>B)can
Q153: The solubility of Ar in water at
Q154: How many grams of solution are present
Q156: A 0.100 m solution of which one
Q157: The principal reason for the extremely low
Q158: Which of the following will have an
Q159: Water (H<sub>2</sub>O)and the alcohol methanol (CH<sub>3</sub>OH)are infinitely
Q160: What is the concentration in ppm of