Multiple Choice
The osmotic pressure of a solution formed by dissolving 45.0 mg of aspirin 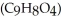 in 0.250 L of water at 25 °C is ________ atm.
in 0.250 L of water at 25 °C is ________ atm.
A) 24.5
B) 2.05 × 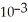
C) 0.0245
D) 4.41
E) 2.48
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q79: Pairs of liquids that will mix in
Q80: Calculate the molarity of a 10.0% (by
Q81: Which of the following would be least
Q82: Which of the following choices has the
Q83: 291.5 grams of calcium chloride is dissolved
Q85: What is the osmotic pressure (in atm)of
Q86: Calculate the molality of a 27.0% (by
Q87: Determine the freezing point (°C)of a 0.015
Q88: What is the molarity of a 7.00%
Q89: Molality is defined as the _.<br>A)moles solute/moles