At Elevated Temperatures,methylisonitrile (CH3NC)isomerizes to Acetonitrile (CH3CN): CH3NC (G)→ CH3CN
Multiple Choice
At elevated temperatures,methylisonitrile (CH3NC) isomerizes to acetonitrile (CH3CN) : CH3NC (g) → CH3CN (g)
The reaction is first order in methylisonitrile.The attached graph shows data for the reaction obtained at 198.9 °C. 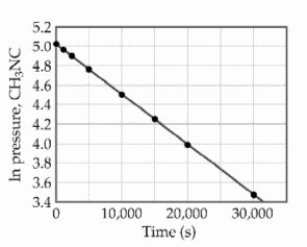 What is the rate constant (s-1) for the reaction?
What is the rate constant (s-1) for the reaction?
A) -1.9 × 104
B) +5.2 × 10-5
C) +1.9 × 104
D) -5.2 × 10-5
E) +6.2
Correct Answer:

Verified
Correct Answer:
Verified
Q72: The reaction A → B is first
Q73: As the temperature of a reaction is
Q74: The reaction 2NOBr (g)→ 2 NO (g)+
Q75: What is the order of the reaction
Q76: The half-life for a first order rate
Q78: It was determined experimentally that the reaction
Q79: Which energy difference in the energy profile
Q80: The overall reaction below is _ and
Q81: What is the average rate of disappearance
Q82: One difference between first- and second-order reactions