Multiple Choice
The half-life of a first-order reaction is 13 min.If the initial concentration of reactant is 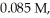 it takes
it takes  for it to decrease to 0.055 M.
for it to decrease to 0.055 M.
A) 8.2
B) 11
C) 3.6
D) 0.048
E) 8.4
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q37: The mechanism for formation of the product
Q38: Of the following,only _ is a valid
Q39: Units of the rate constant of a
Q40: The overall order of a reaction is
Q41: The following reaction is second order in
Q43: Define Beer's Law.
Q44: The concentration of S<sub>2</sub>O<sub>8</sub><sup>2-</sup> remaining at 800
Q45: The magnitude of the rate constant is
Q46: A burning splint will burn more vigorously
Q47: The decomposition of [A] in solution at