Multiple Choice
The rate of disappearance of HBr in the gas phase reaction 2HBr (g) → H2 (g) + Br2 (g)
Is 0.190 M 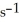 at 150 °C.The rate of appearance of Br2 is ________ M
at 150 °C.The rate of appearance of Br2 is ________ M 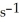 .
.
A) 2.63
B) 0.095
C) 0.0361
D) 0.380
E) 0.436
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q102: The reaction below is first order in
Q103: The rate limiting step in a reaction
Q104: The rate law of a reaction is
Q105: The rate of a second order reaction
Q106: What is the order of the reaction
Q108: For a zero-order reaction,a plot of _
Q109: Biological catalysts are referred to as _.
Q110: The reaction A (aq)→ B (aq)is first
Q111: Of the following,all are valid units for
Q112: The isomerization of methylisonitrile to acetonitrile CH<sub>3</sub>NC