Multiple Choice
The combustion of ethylene proceeds by the reaction C2H4 (g) + 3O2 (g) → 2CO2 (g) + 2H2O (g)
When the rate of disappearance of O2 is 0.13 M 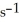 ,the rate of disappearance of C2H4 is ________
,the rate of disappearance of C2H4 is ________ 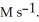
A) 0.087
B) 0.043
C) 0.39
D) 0.20
E) 0.26
Correct Answer:

Verified
Correct Answer:
Verified
Q63: The graph shown below depicts the relationship
Q64: The overall reaction order is the sum
Q65: The rate of disappearance of HBr in
Q66: _ are used in automotive catalytic converters.<br>A)Heterogeneous
Q67: The instantaneous rate of a reaction can
Q69: In general,as activation energy increases,reaction rate _.<br>A)goes
Q70: At elevated temperatures,methylisonitrile (CH<sub>3</sub>NC)isomerizes to acetonitrile (CH<sub>3</sub>CN):
Q71: What is the magnitude of the rate
Q72: The reaction A → B is first
Q73: As the temperature of a reaction is