Multiple Choice
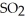
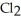 decomposes in the gas phase by the reaction SO2Cl2 (g) → SO2 (g) + Cl2 (g)
decomposes in the gas phase by the reaction SO2Cl2 (g) → SO2 (g) + Cl2 (g)
The reaction is first order in SO2Cl2 and the rate constant is 3.0 × 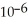
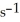 at 600 K.A vessel is charged with 3.6 atm of SO2Cl2 at 600 K.The partial pressure of SO2Cl2 at
at 600 K.A vessel is charged with 3.6 atm of SO2Cl2 at 600 K.The partial pressure of SO2Cl2 at 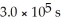 is ________ atm.
is ________ atm.
A) 0.85
B) 3.2
C) 1.5
D) 0.19
E) 9.3 × 104
Correct Answer:

Verified
Correct Answer:
Verified
Q4: A second-order reaction has a half-life of
Q5: Define activation energy.
Q6: If a rate law is first order
Q7: What is the order of the reaction
Q8: The rate constant of a first-order process
Q10: The enzyme _ converts nitrogen into ammonia.<br>A)oxygenase<br>B)hydrogenase<br>C)oxidase<br>D)nitroglycerine<br>E)nitrogenase
Q11: Of the following,_ will lower the activation
Q12: Fe and Mo are transition metals found
Q13: The reaction CH<sub>3</sub>-N≡C → CH<sub>3</sub>-C≡N<br>Is a first-order
Q14: A compound decomposes by a first-order process.If