Multiple Choice
The rate constant for a particular second-order reaction is 0.47 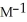
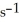 .If the initial concentration of reactant is
.If the initial concentration of reactant is 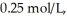 it takes ________ s for the concentration to decrease to
it takes ________ s for the concentration to decrease to 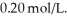
A) 2.1
B) 1.4
C) 1.0
D) 0.47
E) 0.20
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q49: If the rate law for the reaction
Q50: The primary source of the specificity of
Q51: During the combustion of ethylene gas,C<sub>2</sub>H<sub>4</sub>,carbon dioxide
Q52: The average rate of disappearance of A
Q53: A reaction was found to be second
Q55: What value is represented by the y-intercept
Q56: The rate law for a reaction is
Q57: Which one of the following graphs shows
Q58: The rate of a reaction depends on
Q59: A second-order reaction has a half-life of