Multiple Choice
Given the following reaction at equilibrium,if Kc = 5.84 x 105 at 230.0 °C,Kp = ________. 2NO (g) + O2 (g) 
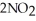 (g)
(g)
A) 3.67 × 10-2
B) 1.41 × 104
C) 6.44 × 105
D) 2.40 × 106
E) 2.41 × 107
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q42: Which of the following expressions is the
Q43: Consider the following reaction at equilibrium: 2N
Q44: The K<sub>eq</sub> for the equilibrium below is
Q45: Which of the following expressions is the
Q46: Which of the following expressions is the
Q48: The K<sub>eq</sub> for the equilibrium below is
Q49: At 1000.0 K,the equilibrium constant for the
Q50: Given the following reaction at equilibrium,if K<sub>p</sub>
Q51: Which one of the following will change
Q52: The equilibrium expression for K<sub>p</sub> for the