Multiple Choice
The value of 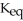 for the following reaction is 0.25: S
for the following reaction is 0.25: S 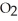 (g) + N
(g) + N 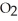 (g)
(g)  S
S 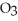 (g) + NO (g)
(g) + NO (g)
The value of 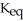 at the same temperature for the reaction below is ________.
at the same temperature for the reaction below is ________.
3S 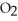 (g) + 3N
(g) + 3N 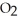 (g)
(g)  3S
3S 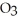 (g) + 3NO (g)
(g) + 3NO (g)
A) 1.6 × 10-2
B) 7.5 × 10-1
C) 8.3 × 10-2
D) 6.4 × 101
E) 0.25
Correct Answer:

Verified
Correct Answer:
Verified
Q18: The equilibrium-constant expression for the reaction Ti
Q19: If a reaction is endothermic,_ the reaction
Q20: If Reaction A + Reaction B =
Q21: Based on Le Châtelier's principle,increasing pressure at
Q22: Which one of the following is true
Q24: Consider the following reaction at equilibrium: 2C
Q25: Le Châtelier's principle states that if a
Q26: How is the reaction quotient used to
Q27: Phosphorous trichloride and phosphorous pentachloride equilibrate in
Q28: In the coal-gasification process,carbon monoxide reacts with