Multiple Choice
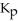 = 0.0198 at 721 K for the reaction 2HI (g)
= 0.0198 at 721 K for the reaction 2HI (g) 
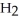 (g) +
(g) + 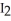 (g)
(g)
In a particular experiment,the partial pressures of 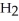 and
and 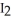 at equilibrium are 0.678 and 0.788 atm,respectively.The partial pressure of HI is ________ atm.
at equilibrium are 0.678 and 0.788 atm,respectively.The partial pressure of HI is ________ atm.
A) 7.87
B) 27.0
C) 5.19
D) 0.103
E) 0.0106
Correct Answer:

Verified
Correct Answer:
Verified
Q70: The value of K<sub>eq</sub> for the equilibrium
Q71: Given the following reaction at equilibrium at
Q72: At equilibrium,_.<br>A)all chemical reactions have ceased<br>B)the rates
Q73: At 24°C,K<sub>p</sub> = 0.080 for the equilibrium:
Q74: The effect of a catalyst on an
Q76: Fritz Haber was awarded the _ Nobel
Q77: If the value for the equilibrium constant
Q78: The K<sub>eq</sub> for the equilibrium below is
Q79: If the reaction quotient Q for a
Q80: In an experiment,0.42 mol of CO and