Multiple Choice
Using the data in the table,which of the conjugate acids below is the weakest acid? 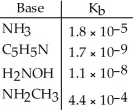
A) NH4+
B) C5H5NH+
C) H3NOH+
D) NH3CH3+
E) NH4+ and NH3CH3+
Correct Answer:

Verified
Correct Answer:
Verified
Q84: K<sub>a</sub> for HF is 7.0 × 10<sup>-4</sup>.K<sub>b</sub>
Q85: Classify the following compounds as weak acids
Q86: A 1.0 × 10<sup>-2</sup> M aqueous solution
Q87: Classify the following compounds as weak acids
Q88: The conjugate acid of SO<sub>4</sub><sup>2-</sup> is _.<br>A)OH<sup>-</sup><br>B)H<sub>2</sub>SO<sub>4</sub><br>C)HSO<sub>4</sub><sup>-</sup><br>D)HSO<sub>4</sub><sup>2-</sup><br>E)H<sub>3</sub>SO<sub>4</sub><sup>+</sup>
Q90: Determine the pH of a 0.35 M
Q91: What is the pH of an aqueous
Q92: Using the data in the table,which of
Q93: Which of the following salts will produce
Q94: Of the following substances,an aqueous solution of