Multiple Choice
The pH of an aqueous solution at 25.0 °C is 10.55.What is the molarity of 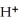 in this solution?
in this solution?
A) 2.8 × 10-11
B) 3.5 × 10-4
C) 3.45
D) 1.1 × 10-13
E) 3.5 × 1010
Correct Answer:

Verified
Correct Answer:
Verified
Q73: Which one of the following is the
Q74: Using the data in the table,which of
Q75: Hydrochloric acid is a strong acid.This means
Q76: The conjugate base of NH<sub>3</sub> is _.<br>A)NH<sub>2</sub><sup>-</sup><br>B)NH<sub>4</sub><sup>+</sup><br>C)NH<sub>2</sub>OH<br>D)H<sub>3</sub>O<sup>+</sup><br>E)OH<sup>-</sup>
Q77: Which of the following 0.5 M aqueous
Q79: The <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="The for
Q80: The K<sub>a</sub> for HCN is 4.9 ×
Q81: An aqueous solution at 25.0°C contains [
Q82: What is the pH of an aqueous
Q83: Of the following acids,_ is a strong