Multiple Choice
What is the concentration (in M) of hydronium ions in a solution at 25.0 °C with pH = 4.146?
A) 4.15
B) 9.85
C) 1.40 × 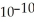
D) 7.15 × 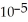
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Q114: The pH of a 0.15 M aqueous
Q115: The pH of a 0.25 M aqueous
Q116: The <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="The for
Q117: An aqueous solution contains 0.10 M HNO<sub>3</sub>.
Q118: Classify the following compounds as weak acids
Q120: Classify the following compounds as weak acids
Q121: Calculate the pOH of a solution at
Q122: The conjugate base of HPO<sub>4</sub><sup>2-</sup> is _.<br>A)PO<sub>4</sub><sup>3-</sup><br>B)H<sub>2</sub>PO<sub>4</sub><br>C)H<sub>3</sub>PO<sub>4</sub><br>D)H<sub>2</sub>PO<sub>4</sub><sup>-</sup><br>E)none
Q123: In the reaction<br>BF<sub>3</sub> + F<sup>-</sup> → BF<sub>4</sub><sup>-</sup><br>BF<sub>3</sub>
Q124: Which of the following aqueous solutions has