Multiple Choice
The acid-dissociation constant at 25.0 °C for hypochlorous acid (HClO) is 3.0 × 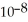 .At equilibrium,the molarity of
.At equilibrium,the molarity of 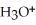 in a 0.066 M solution of HClO is ________.
in a 0.066 M solution of HClO is ________.
A) 4.4 × 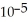
B) 0.066
C) 2.2 × 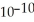
D) 4.35
E) 1.18
Correct Answer:

Verified
Correct Answer:
Verified
Q26: Which one of the following is a
Q27: According to the Arrhenius concept,an acid is
Q28: Which of the following salts will produce
Q29: Classify the following compounds as weak bases
Q30: What is the conjugate acid of NH<sub>2</sub><sup>-</sup>?<br>A)NH<sub>2</sub><sup>+</sup><br>B)NH<sub>3</sub><sup>+</sup><br>C)NH<sub>4</sub><sup>+</sup><br>D)NH<sub>3</sub><br>E)NH<sub>4</sub>OH
Q32: What is the pOH of an aqueous
Q33: An aqueous basic solution has a concentration
Q34: Calculate the pH of a solution at
Q35: The <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="The for
Q36: A 0.5 M solution of _ has