Multiple Choice
In which of the following aqueous solutions does the weak acid exhibit the highest percentage ionization?
A) 0.01 M H2SO3 ( 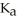 = 1.4 ×
= 1.4 × 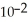 )
)
B) 0.01 M HCN ( 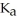 = 6.2 ×
= 6.2 × 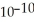 )
)
C) 0.01 M H2CO3 ( 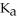 = 4.5 ×
= 4.5 × 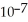 )
)
D) 0.01 M HC3H5O2 ( 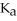 = 1.3 ×
= 1.3 × 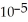 )
)
E) 0.01 M HOCl ( 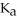 = 3.5 ×
= 3.5 × 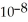 )
)
Correct Answer:

Verified
Correct Answer:
Verified
Q11: HA is a weak acid.Which equilibrium corresponds
Q12: What is the pH of an aqueous
Q13: The K<sub>a</sub> of some acid,HA,at 25.0 °C
Q14: Using the data in the table,which of
Q15: An aqueous solution of a particular compound
Q17: What is the pOH of 0.606 M
Q18: What is the conjugate acid of OH<sup>-</sup>?<br>A)O<sub>2</sub><br>B)H<sub>2</sub>O<br>C)O<sup>-</sup><br>D)O<sup>2-</sup><br>E)H<sub>3</sub>O<sup>+</sup>
Q19: What is the concentration (in M)of hydroxide
Q20: Calculate the concentration (in M)of hydroxide ions
Q21: A 0.10 M aqueous solution of the