Multiple Choice
What is the pH of a 0.40 M aqueous solution of N 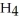 Br at 25.0 °C?
Br at 25.0 °C? 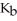 for N
for N 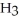 is 1.8 ×
is 1.8 × 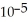 .
.
A) 4.82
B) 2.57
C) 9.18
D) 11.43
E) 11.23
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q43: Which of the following aqueous solutions has
Q44: The pH of a 0.60 M aqueous
Q45: Which of the following salts will produce
Q46: The molar concentration of hydronium ion in
Q47: Of the compounds below,a 0.1 M aqueous
Q49: Calculate the concentration (in M)of hydronium ions
Q50: What is the [ <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="What
Q51: The pOH of a 0.10 M solution
Q52: Of the following,which is the strongest acid?<br>A)HIO<sub>4</sub><br>B)HIO<sub>3</sub><br>C)HIO<sub>2</sub><br>D)HIO<br>E)The
Q53: An aqueous solution of ammonia at 25.0